
The history of the research group starts in the 90s with the study of the mechanisms underlying the mucoadhesion phenomenon and with the investigation of the interaction between mucins, the main component of mucus, and some natural polymers such as cellulose derivatives, chitosan and its derivatives, and hyaluronic acid. Among these, the multifunctional polymers proved also to interact with the underlying epithelium by disturbing the intercellular junctions and acting as penetration enhancers or by promoting the cell proliferation and enhancing tissue repair. In 2000s the national grants received and the collaboration with researchers of IRCCS San Matteo Hospital (Pavia) have directed the research of the group on the use of such multifunctional and bioactive polymers for the development of therapeutic platforms intended for tissue repair, focusing in particular on the treatment of mucosa lesions, skin ulcers, tendons damages and nerve injuries.
Currently the research activities are focused on the design and the development of multifunctional platform to enhance and promote tissue reparation and to prevent or treat microbial infections. The research group possesses a deep expertise in microparticle/nanoparticles development including polymeric and lipidic systems, and moreover in nano/microfiber production.
Design and development of engineered
tendon substitutes
Development of bioadhesive and/or in
situ gelling systems
Design and development of composite
systems in the treatment of
glioblastoma multiforme
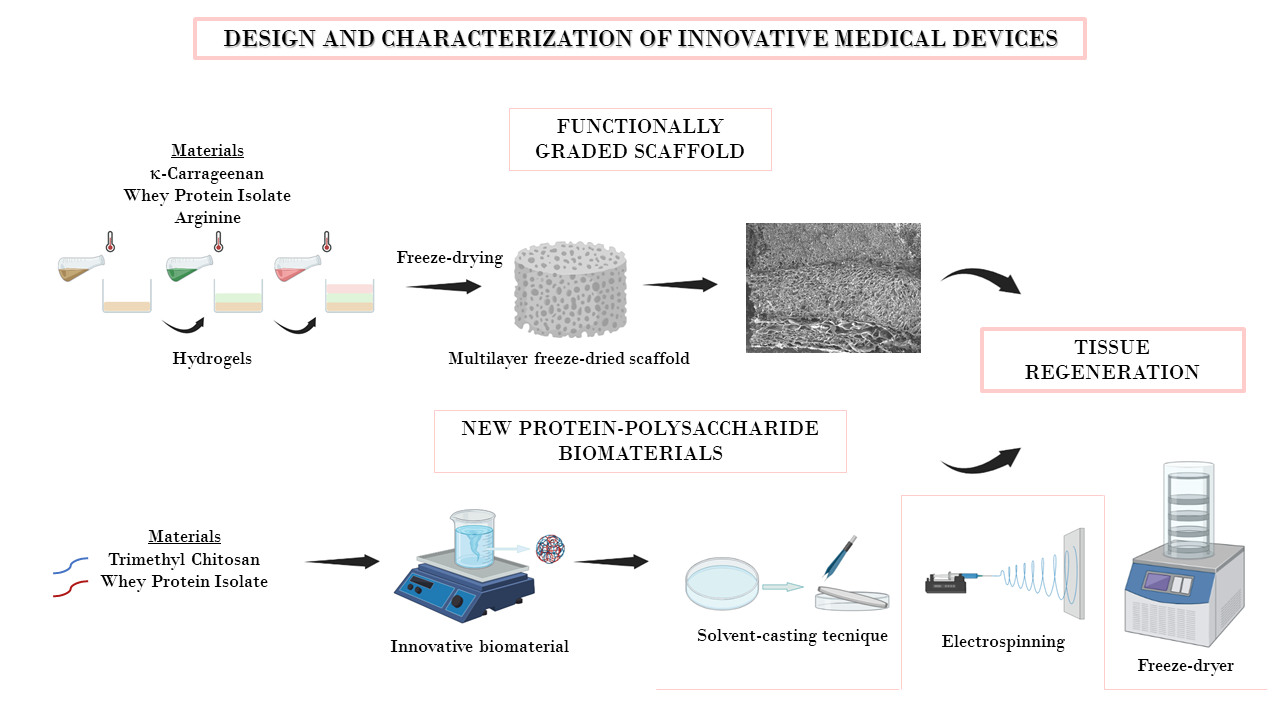
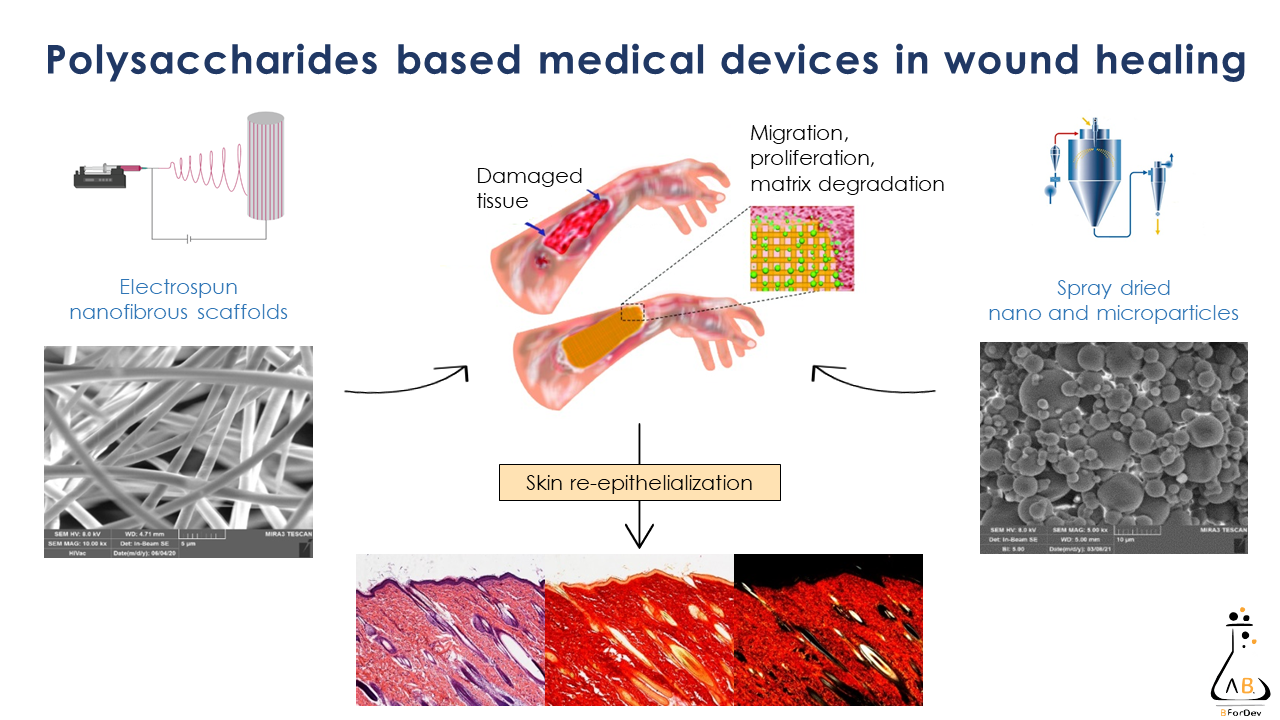
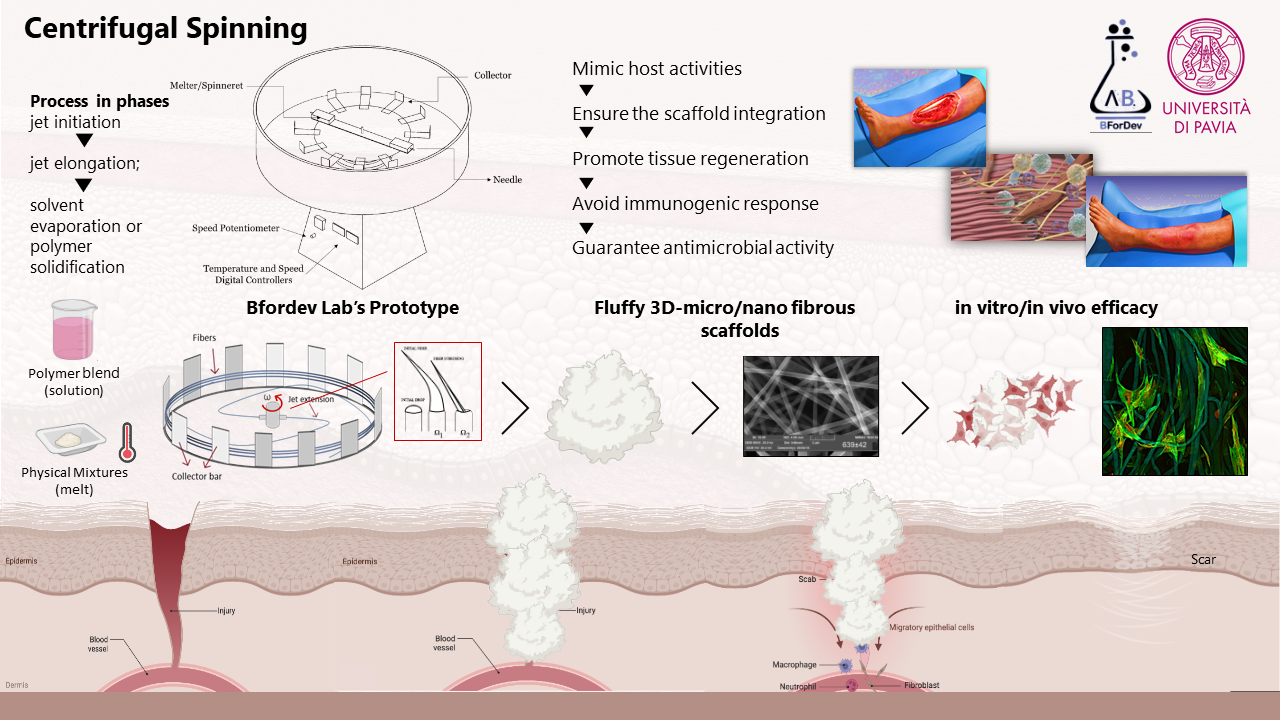
Aim of this research is to design and develop flexible therapeutic platforms to enhance wound healing and to prevent or treat infection in chronic wounds. Scaffolds are prepared by means of easy scalable processes, as electrospinning, centrifugal spinning, and spray-drying; porous scaffolds produced by freeze-drying are also under investigation. These systems are designed to have a three-dimensional structure capable of homing cells and of enhancing their proliferation and extracellular matrix production to restore the native tissue. Green manufacturing processes are under development to avoid organic solvents and waste. Moreover, part of the activities is devoted to the study of innovative materials to obtain scaffolds characterized by suitable mechanical properties and complete in vivo degradation. Functional materials with advanced properties for regenerative medicine are used: in particular, polysaccharides, such as chitosan, gums, alginate, maltodextrin, dextran, and carrageenan are employed as biomaterials to enhance healing process thanks to their biocompatibility, low toxicity, and bioactivity. The biological augmentation due to biomodulators, as growth factors, is also used as additional strategy along with the loading of antimicrobial agents (among this also inorganics) are considered to enhance system effectiveness. Design and development of therapeutic platforms for the treatment of nervous tissue injuries.
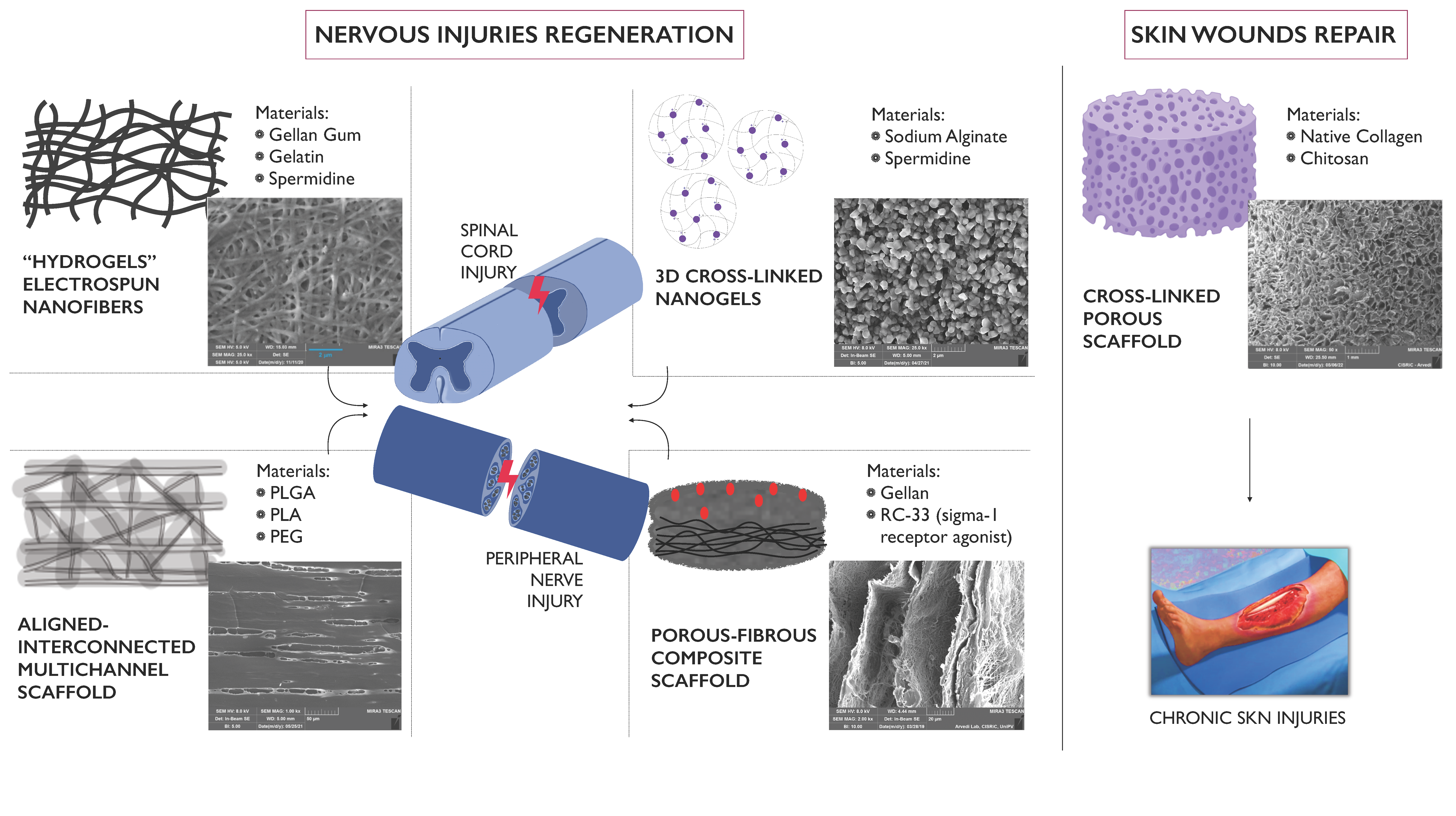
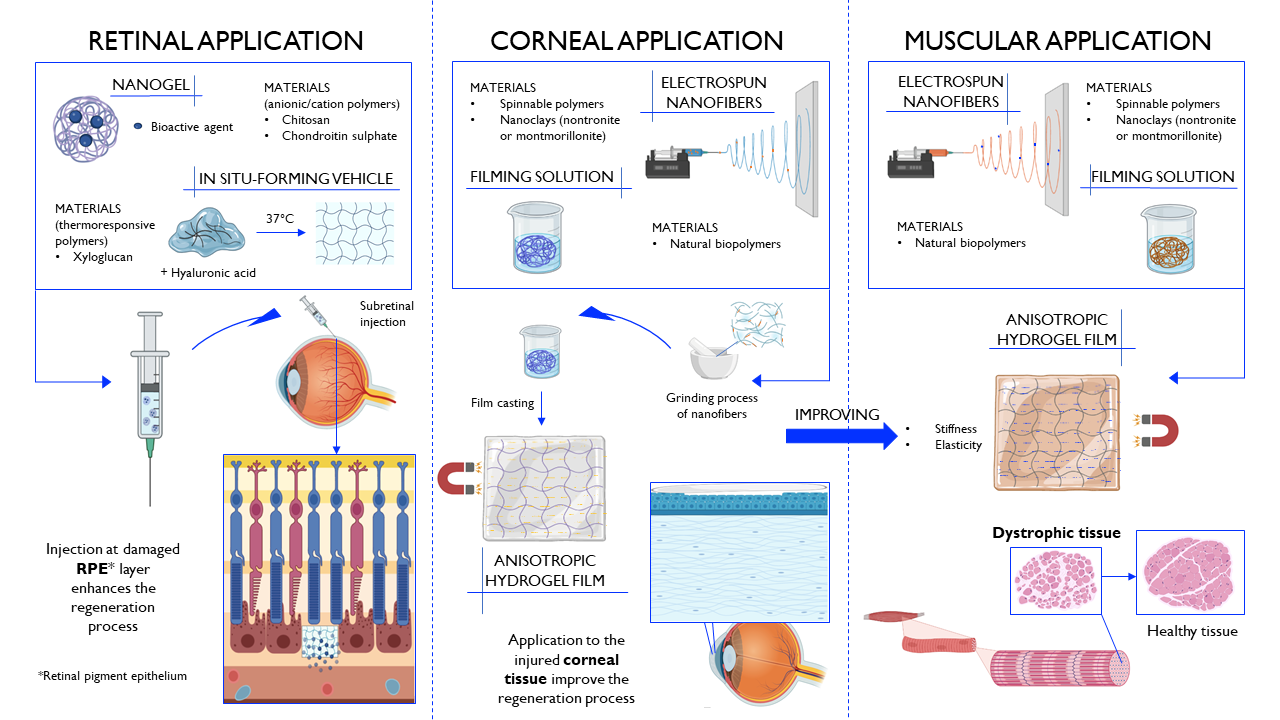
The aim of the research is the development of innovative therapeutic systems, endowed with both neuroprotective and neuroregenerative potential, to be applied at the site of nervous tissue injury. Such systems, thanks to their fibrous and/or multi-channel structure, mimics the architecture of the extracellular matrix, providing mechanical and trophic support to nerve cells and, thus, promoting the recovery and reorganization of neuronal connectivity. Structures characterized by porosity and channels of different dimensions are developed depending on the lesion size and the nervous tissue (central or peripheral) to be treated. Moreover, the developed therapeutic platforms can be used as delivery systems of drug candidates or biological molecules capable of reducing glutamatergic excitotoxicity, preventing oxidative damage and modulating the overexpression of pro-inflammatory cytokines at the site of injury.
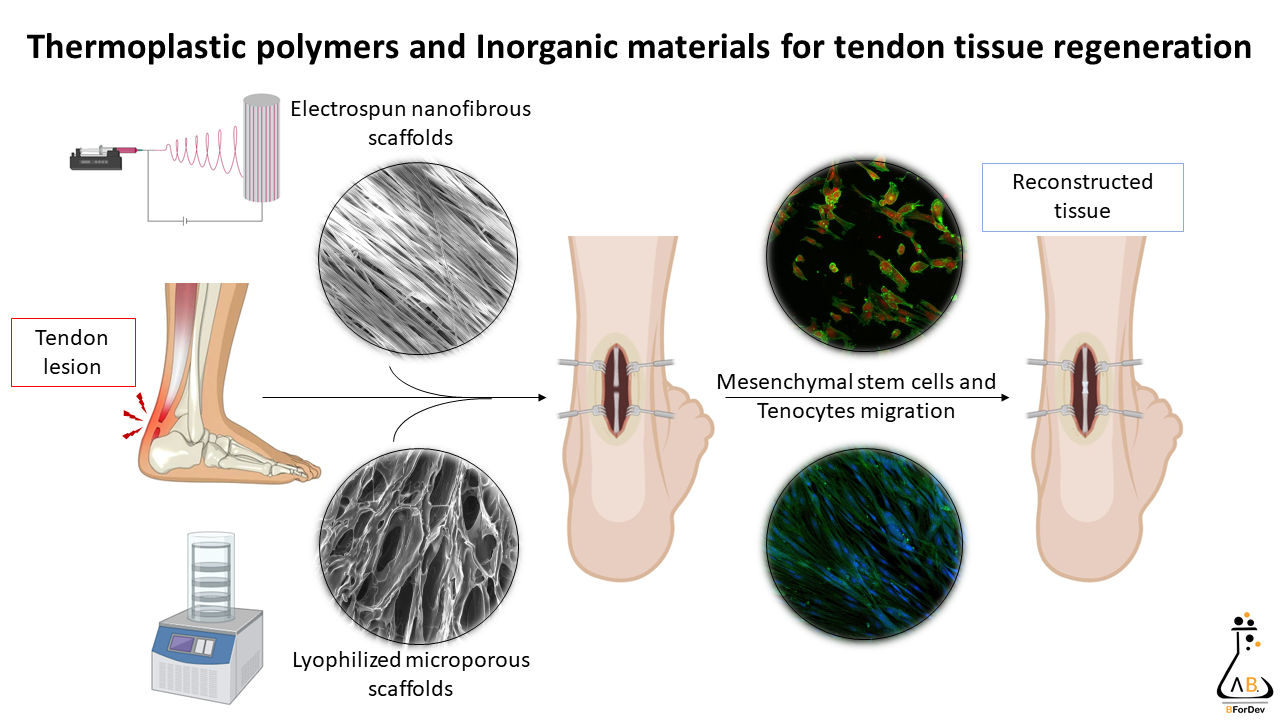
The research focuses on the development of biopolymers-based scaffolds, both natural and synthetic, which have the ability to mimic the structural, biomechanical, and biochemical functions of the extracellular matrix, consequently mimicking the native tissues. The scaffolds are produced by means of the electrospinning, a simple, versatile, flexible, and cost-effective method to spin polymeric materials by means of a high voltage electric field to generate ultra-thin fibers with diameters in the nanometric or micrometric range. In particular, electrospinning allows the generation of nanofibrous porous scaffolds suitable for the tendon tissue regeneration. Prototypes obtained using freeze drying are also under development. Moreover, the polymers are combined with various organic and inorganic materials in order to increase the systems mechanical properties and biocompatibility, enhancing the cell adhesion, proliferation and differentiation, and, consequently, the tissue healing potential.
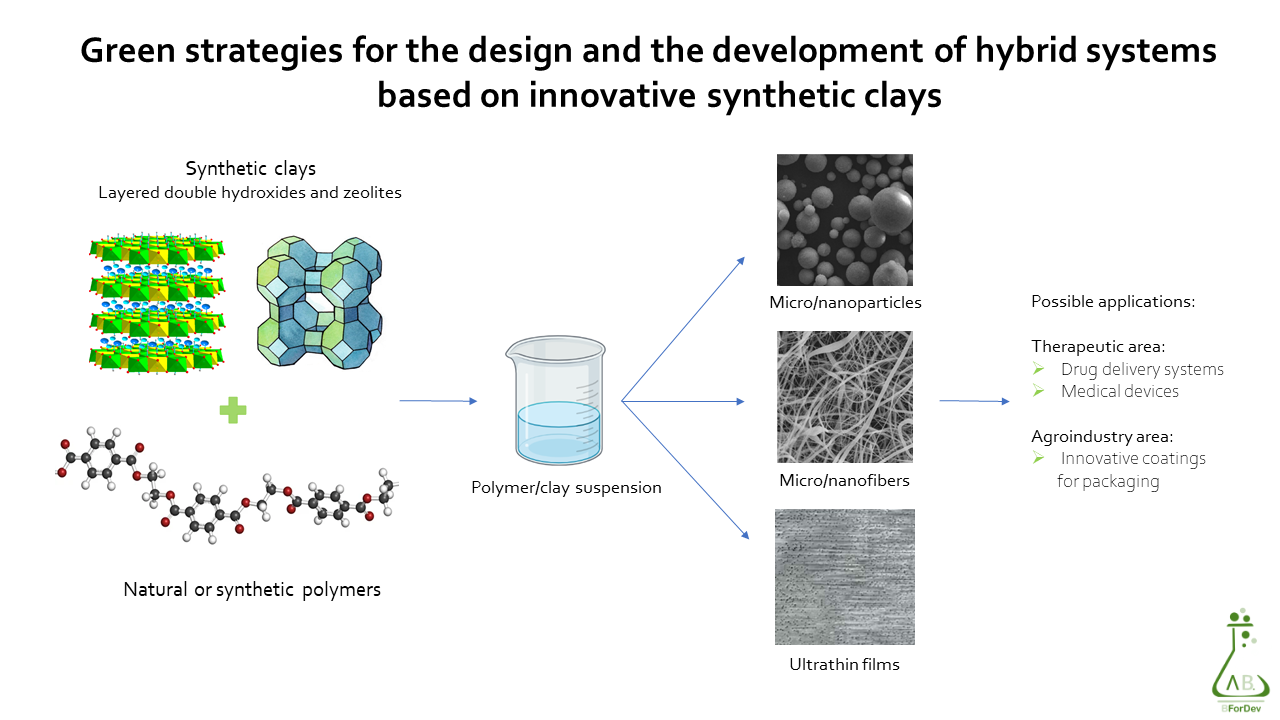
The aim of the research is the development and characterization of bioadhesive and in situ gelling systems for the local treatment of mucosal inflammation and/or infections. Depending on both the mucosa to be treated (buccal, esophageal, vaginal, intestinal) and the pathological condition to solve, the formulations are designed to be used as delivery systems of antimicrobial agents, antiinflammatory compounds and/or probiotics.
The aim of the research is the design of composite systems to be locally applied in the tumor resection site. These systems are composed of lipid nanoparticles, loaded with lipophilic antiproliferative drug candidates and embedded into a polymeric matrix (electrospun fibers or in situgelling and mucoadhesive formulations) of easy application. The gradual degradation/dissolution of the polymeric matrix in the biologic fluids should ensure a controlled release of the nanosystems, which, thanks to their size and surface properties, should be able to establish an intimate contact with the remaining cancer cells.
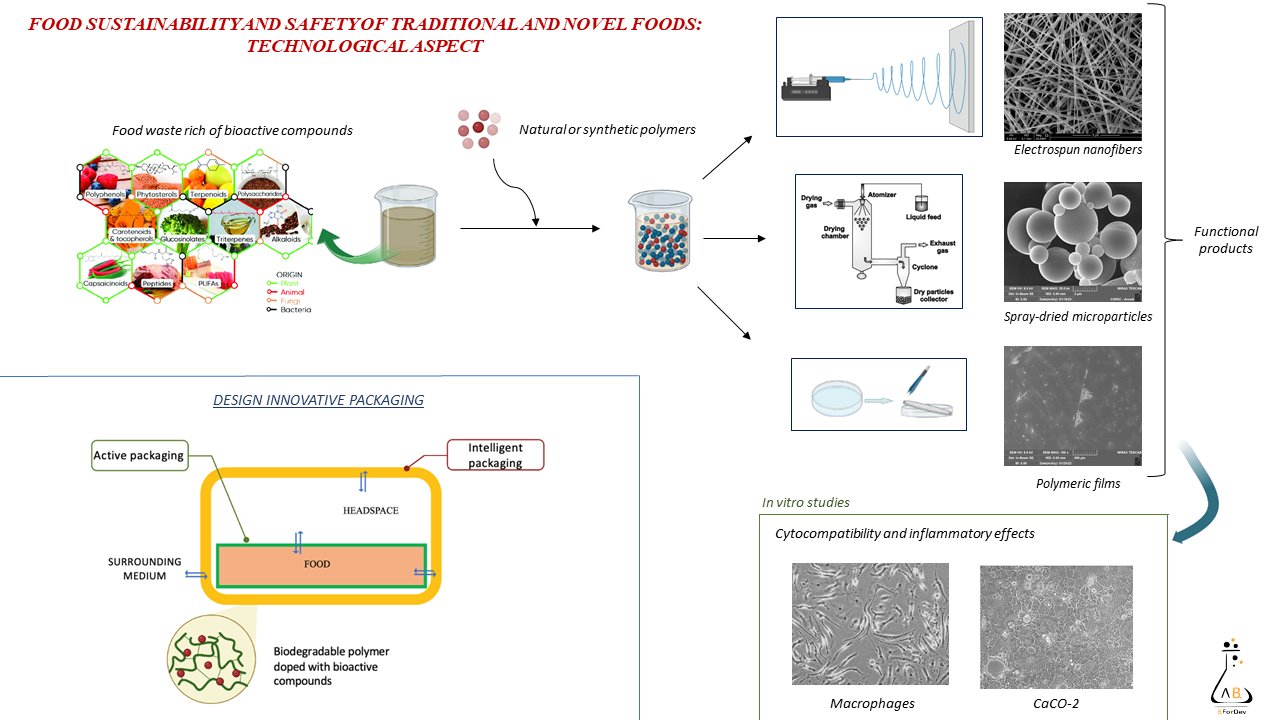
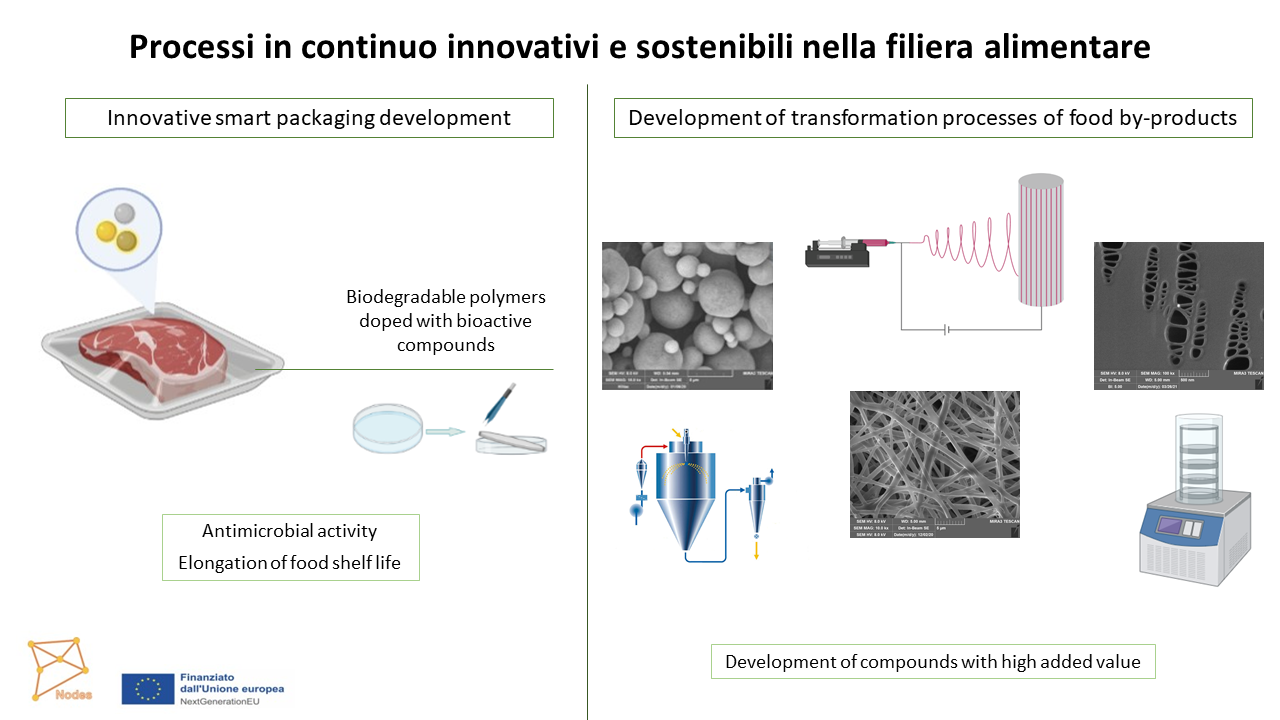
The aim is to design and develop smart packaging based on polymeric films or fibers to adsorb volatile compound and simultaneously to release antioxidants to increase food shelf life
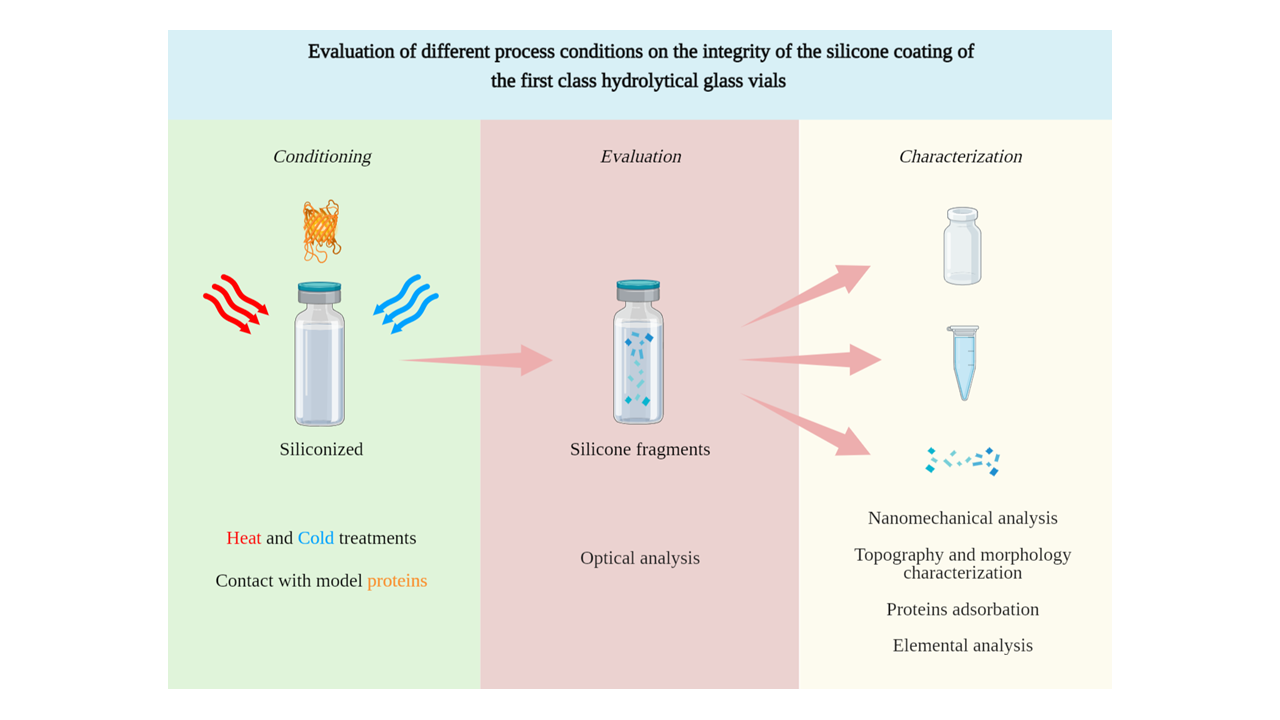
The aim is to investigate the evolution of glass vials, as primary packaging for injectables, subjected to the routine manufacturing processes (sterilization and free drying).
