
This project has been funded by the Italian Ministry of University and Research (MUR) under the PRIN 2022 programme.


The MASTER project aims to develop a smart and biocompatible device based on magnetic-responsive scaffolds designed to promote tendon regeneration.
These 3D-printed porous systems combine thermoplastic polyurethane with bioactive compounds and magnetic particles to stimulate cell activity and healing processes under magnetic field exposure.
The innovation lies in mimicking the complex tendon-to-bone interface both structurally and biomechanically, enabling localized and tunable therapeutic responses.
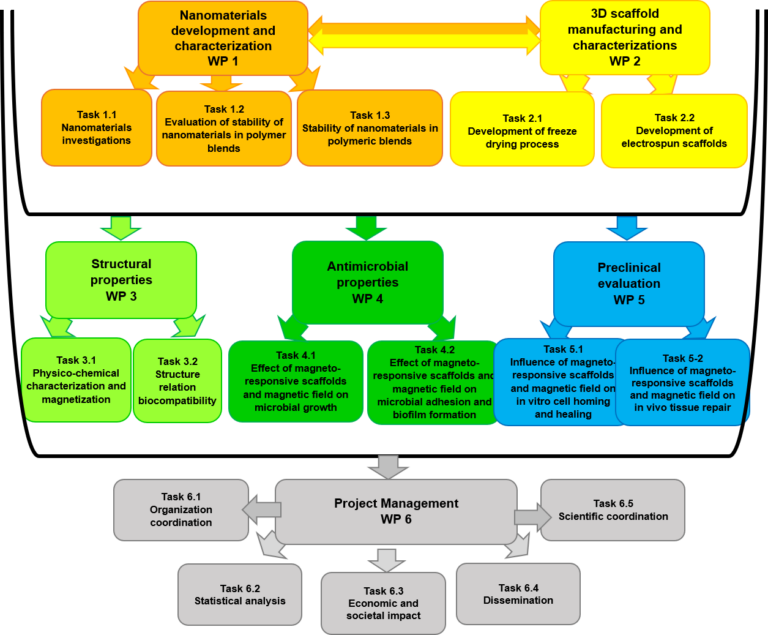
The project involves the design and development of highly porous 3D scaffolds based on thermoplastic polyurethane. These scaffolds are doped with chondroitin sulfate (CS) and casein phosphopeptides (CPPs), aiming to mimic the structural, biomechanical, and biochemical properties of the tendon-to-bone interface (TBI).
The materials and processing steps are tailored to support cell adhesion, mechanical strength, and biological integration, following a modular and progressive structure across work packages.
Tendon injuries frequently require surgical treatment, and, despite recent advancements in orthopedic surgery, they yield less than optimal results and present the risk of microbial colonization, since the devices used could trigger biomaterial-related infections and biofilm formation. Furthermore, healed tendons tend to form scar tissue with different mechanical properties than healthy ones and are prone to re-injury due to their lack of native tissue complexity.
Since tendons are force transmitters and mechanoresponsive tissues, cells within the tendon tissues perceive a complex microenvironment where both environmental cues and cellular mechanotransduction are pivotal in different cell responses, such as apoptosis, proliferation, migration, and differentiation.
Recently, biomechano-responsive materials are emerging in tendon tissue engineering. The synergism of stimuli-responsive biomaterials and mechanical stimuli seems fundamental as teno-inductive cues to boost tendon healing. From this perspective, magneto-responsive medical devices (as tendon substitutes) and magnetotherapy should serve as mediators of mechano-transduction to enhance tendon regeneration.
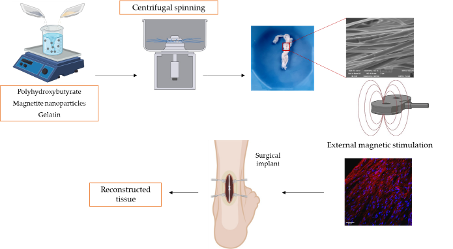
The aim of the project is the design and the development of biomechanic-responsive 3D scaffolds able to improve surgical repair or reconstruction of tendons/ligaments. Thermoplastic polymers (polyurethane and polyacrylcyanoacrylate) will be associated with chondroitin sulfate to obtain slow reabsorption (degradation) to guarantee the scaffolds to support the mechanical load until the regeneration of the new tissue is completed.
Lyophilized (sponge-like) or electrospun scaffolds will be doped with magnetic iron oxide nanomaterials to obtain a hierarchical structure, responsive to magnetotherapy. The activation of the iron oxide nanomaterials should act as a mediator of mechanotransduction to remotely deliver mechanical stimulation directly to the cells. This should boost tendon healing by achieving teno-inductive cues. Magnetite nanoparticles, the benchmark, and Fe-Mg-hydrotalcites will be compared to evaluate the in vitro efficacy and safety profiles.
The 3D scaffolds will be manufactured using both freeze-drying and electrospinning, well-known techniques to obtain hierarchically structured scaffolds for tissue engineering. The two approaches will enable the production of different structures. The sponge-like matrix will be an interconnected structure with pores while the nanofibrous scaffolds will consist of entangled fibers given a certain porosity. Considering these, the mechano stimulation could be differently transmitted to the surrounding and to the infiltrated cells depending on the structure stiffness.
Moreover, the reproducibility and the robustness of the manufacturing procedure and the identification of the quality attributes to assure reproducibility of the scaffold will be considered.
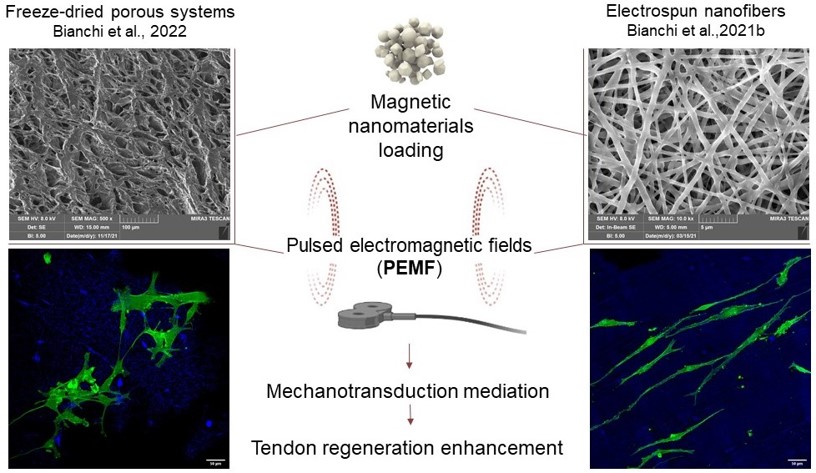
Visual representation of the experimental design and magnetic scaffold application in tendon regeneration.
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's
Lorem Ipsum is simply dummy text of the printing and typesetting industry. Lorem Ipsum has been the industry's standard dummy text ever since the 1500s
The project is coordinated by the University of Pavia (UNIPV), in collaboration with three other Italian universities.
UNIPV acts as the lead institution and manages both project implementation and dissemination activities.

UNIPV is the project coordinator and will provide expertise in the formulation of 3D electrospun scaffolds doped with nanomaterials for tissue engineering. Moreover, UNIPV has expertise in the characterization of in vitro preclinical performance of inorganic and polymers based systems. The recent research activities allowed to gain experience in the characterization of the influence of topography and composition on cell adhesion (tenocytes/osteoblasts) and ECM production and on stem cell differentiation. Moreover, UNIPV possesses deep knowledge on the employment of inorganics (HP, Ag NP and clay minerals) in tissue engineering, and their synergic activity in wound healing. For more than 15 years, UNIPV has closely collaborated with medical doctors belonging to Fondazione IRCCS Policlinico San Matteo (S. Matteo Hospital) (Pavia, Italy) in tissue engineering.

UMIL will provide expertise in the biophysical characterization of magnetic responsive nanomaterials and 3D scaffolds.
UMIL expertise covers the biophysics of colloidal nanostructured systems in solution, lipid membranes, peptides and proteins, and vectors for drug delivery and targeting, also in interaction with biomimetic model membranes and surfaces.
The biologist component possesses the required knowledge on tendon and bone cell biology, as well as the biochemical ability to set up new assays and in vitro cell culture and differentiation protocols.
Within the present project, UMIL will provide expertise and technical skills in the study of magnetic, structural, dynamic and interaction properties of magnetic responsive nanomaterials and magnetic scaffolds by complementary experimental techniques: laser light, synchrotron radiation and neutron scattering, neutron reflectometry, calorimetry, and on the evaluation of biocompatibility and the in vitro efficacy of the magnetic 3D scaffolds at cellular levels.

POLITO will be mainly involved in the setup of the freeze-drying process and in the development and the bio-characterizations of the 3D scaffolds.
The team applies the principles of molecular science and engineering to explore pharmaceutical systems on the molecular level. Through sophisticated computational and experimental methods, it develops new technologies, generally applicable for the engineering of high-value pharmaceutical/medical applications. Special emphasis is currently placed on stabilization and formulation of bioproducts, crystallization of small and large molecules, continuous manufacturing in the pharmaceutical industry, development of innovative eco-friendly polymerization systems, controlled drug release, and design of large, complex scaffolds with controlled micro- and nano-architecture.
As regards this last activity, which is of interest for the present project, POLITO has developed new freezing protocols and methods which, coupled with the freeze-drying process, made it possible to create porous media with a controlled microarchitecture. These techniques, in addition to the use of photopolymerization in emulsion and spray freeze-drying, have been used for the development of scaffolds for bone implants, and porous micro and NP with a customized structure (full and porous nanoparticle and nanocapsules) which allows controlling the release rates of various drugs.

UNITO will be involved in the antimicrobial and antibiofilm properties of the 3D magneto-responsive scaffolds. The team focuses its activities mainly on:
a) investigation of drug characteristics on primary functions of phagocytes (such as intracellular killing activity, cytokine release pattern, apoptosis, ROS production) to determine their immunomodulatory properties;
b) direct antimicrobial activity of oxygen nanobubbles/nanodroplets, loaded with antimicrobial agents, as vectors for the treatment of cutaneous microbial infections in immunosuppressed patients.
Furthermore, within the topic of the present project, the UNIT is involved in various multidisciplinary projects with chemists (UNITO), engineers (POLITO), and orthopaedic surgeons (CTO hospital, Turin) aimed to study the chemico-physical and morphological characteristics of different biomaterials (i.e. titanium alloys, cements, polymers), as orthopaedic scaffolds, functionalized with either biphasic calcium phosphates (i.e. hydroxyapatite, β-tricalcium phosphate) and antimicrobials (i.e. Ag+) that could influence microbial adhesion and subsequent biofilm formation.
UNITO within the present project will provide expertise and technical skills in the evaluation of the direct effect of inorganic components and magnetic field on microbial growth, by MIC and killing curve determination, and the effect of the 3D scaffold features (e.g. morphology and doping of antimicrobial compounds) on microbial adhesion and biofilm formation.
e-mail: g.sandri@unipv.it
tel: 03829687728
Department of Drug Sciences,
University of Pavia
e-mail: elena.delfavero@unimi.it
tel: 02503 30312
Department of Medical Biotechnology and
Translational Medicine,
University of Milano
e-mail: roberto.pisano@polito.it
tel: +39 0110904679 / 4679
Department of Applied Science and Technology (DISAT),
Politecnico of Torino
e-mail: narcisa.mandras@unito.it
tel: 0116705645
Department of Sciences of Public Health and Pediatrics,
University of Torino
